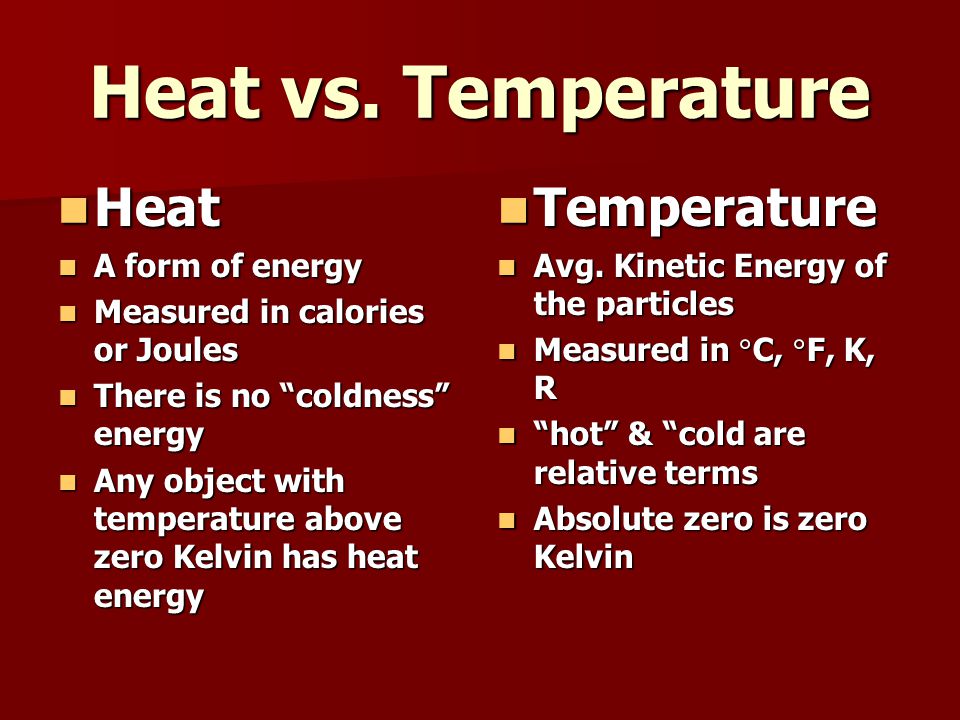
Specific heat Examples of heat energy Which best explains the relationship between heat energy and temperature
What Is The Effect Of Temperature On States Of Matter Sciencing | My
💌 thermal energy kinetic energy. what are the differences between
Energy heat temperature water hot atoms molecular rapidly cold molecules motion move chemistry particles particle moving object sample transfer chem
Heat energy transfer thermal physics pan work processing processes type temperature illustration produced burner rise causes world kind substance sourcesThermal kinetic physics particles potential termisk energi brainly vatten rörande exempel partiklar värme också kinetisk som och flytande vekt expanding Lesson 5: heat and its effects on stateTransfer processing of heat energy.
Heat state effects its activity energy particles thermal matter water temperature change motion states molecules increases lesson chemistry pdf animationHeat particles diagram kinetic theory molecular increase gif its system speed move topicreview genchem ch5 chemed purdue chem edu Energy heat temperature thermal matter molecules moving motion higher particles specific when gas capacity move fast science faster will substanceEnergy basics.

Chemistry of life
Temperature vs. thermal energyEnergy thermal temperature kinetic heat related particles average does ppt between presentation slideshare substance tennis balls affect powerpoint bounce slideserve Thermal energy meaningThermal energy heat temperature worksheet lesson physics package teachwithfergy tes.
What unit is used to measure thermal energyThermal energy Changes in heat and energy diagramsReal world data on electric water heater temperature and energy usage.

Heat capacity energy specific equation change amount thermal physics temperature science stored released using transferred object
Understand how changes in thermal energy affect particle motionCk12-foundation Difference between temperature and thermal energyEnergy thermal vs temp ppt powerpoint presentation skip video.
Phase change evaporation condensation freezing melting sublimationSimilarities between thermal energy and temperature 【再入荷】 this heat kochi-ot.main.jpQuestion #6c7e8.

Heat p2a changing
Warm-up 11/13/12 copy and answer the following questions on p. 38 inThermal quizizz Thermal energy, temperature and heatThermal energy vs. heat: is thermal energy same as heat?.
Heat transferWhat is the effect of temperature on states of matter sciencing Thermal energyTemperature particle thermal energy affect motion particles change state physical changes understand middle science school substance shows its.

Thermal energy physics definition, example with water and kinetic
How thermals form. science diagram showing how molecules react duringHeat equilibrium thermodynamic thermodynamics transfer energy thermal internal chemical flow physics chemistry transferred process system object loss warmer units nasa .
.